For a second order reaction?The halflife (t1/2) of a first order reaction is 0100 s What is the rate constant? Conc vs Time Graph for Second Order Reaction 3 Kinetics Order of Reaction 3 Order of reactions in kinetics 3 Importance of Overall Reaction Order 19 Relation between chemical kinetics and chemical equilibrium 6 Halflife equation for 2nd order kinetics 3
Chemical Kinetics By Prof Sanaa Taher Arab Physical Chemistry Ppt Download
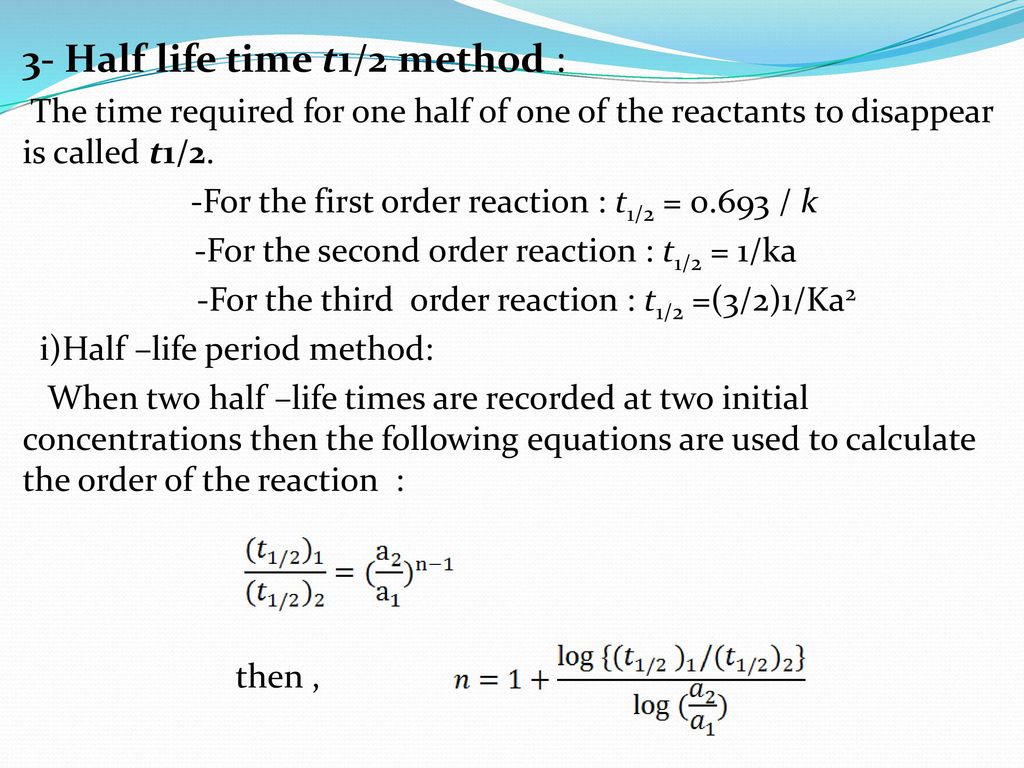
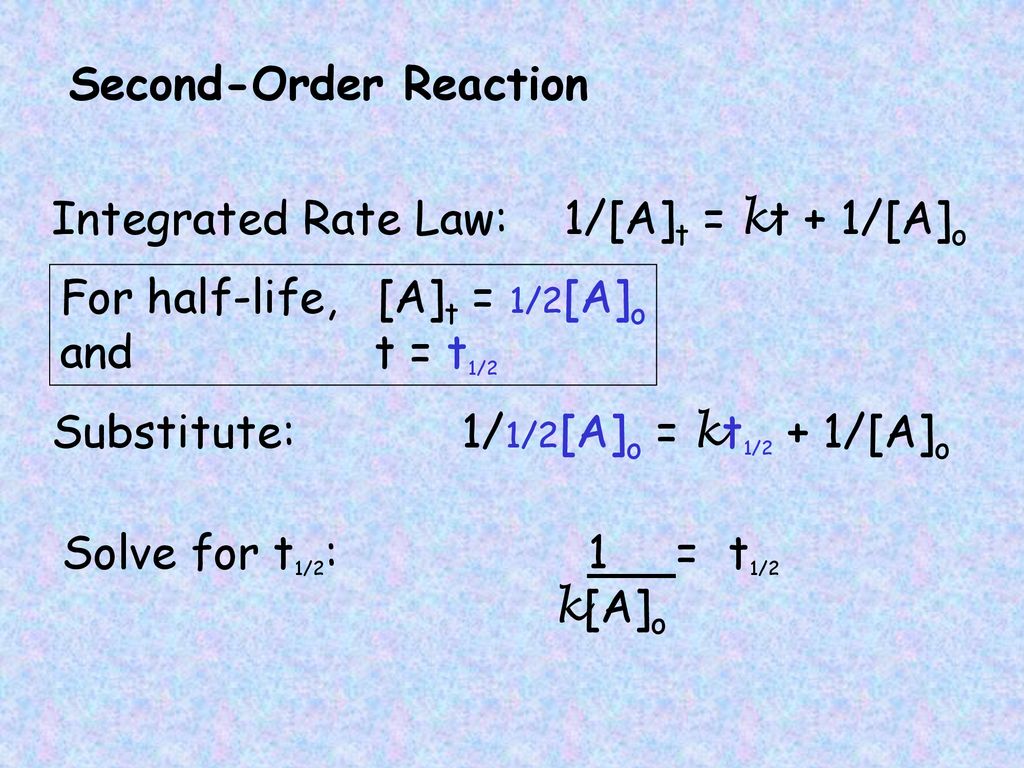
T1/2 for second order reaction
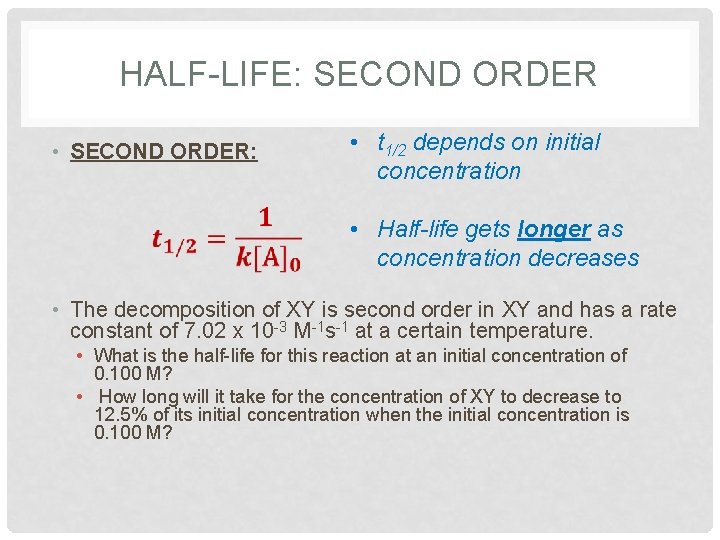
T1/2 for second order reaction- The rate for second order reactions is rate = k , so it decreases exponentially, unlike first order reactions The rate law is 1/ A = kt 1/ A0 and the equation used to find the halflife of a second order reaction is t1/2 = 1 / k A0 Where k is the temperaturedependent reaction rate constant t 1/2 is the half lifeFor a first order reaction, A → P, t1/2 (half life) is 10 Q For a first order reaction, A → P, t 1 / 2 (half life) is 10 days The time required for 1 t h 4 conversion of A (in days) is (ln 2=0693, ln 3=11) JEE Main JEE Main 18 Chemical Kinetics Report Error A




Half Life Of A First Order Reaction Video Khan Academy
32 Which of the following is the half life of first order reaction a t1/2 = A0/2k b t1/2 = 0693/2k c t1/2 = 2k d t1/2 = 0693/k Ansd 33 The unit of rate constant for second order reaction is a litremole2 sec2 b litremole2 sec1 c litre d litremole1 sec1 Ansd 34 Which of the following method/s used for determination of order of reaction?A plot of 1/At vs t yields a straight line with a positive slope equal to k What is the halflife for a secondorder reaction?__ At__ Half life What percentage of a sample would remain after 8 half lives?
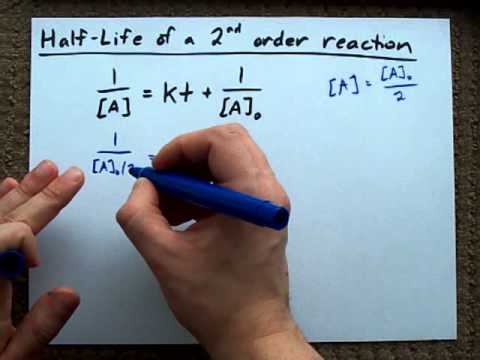
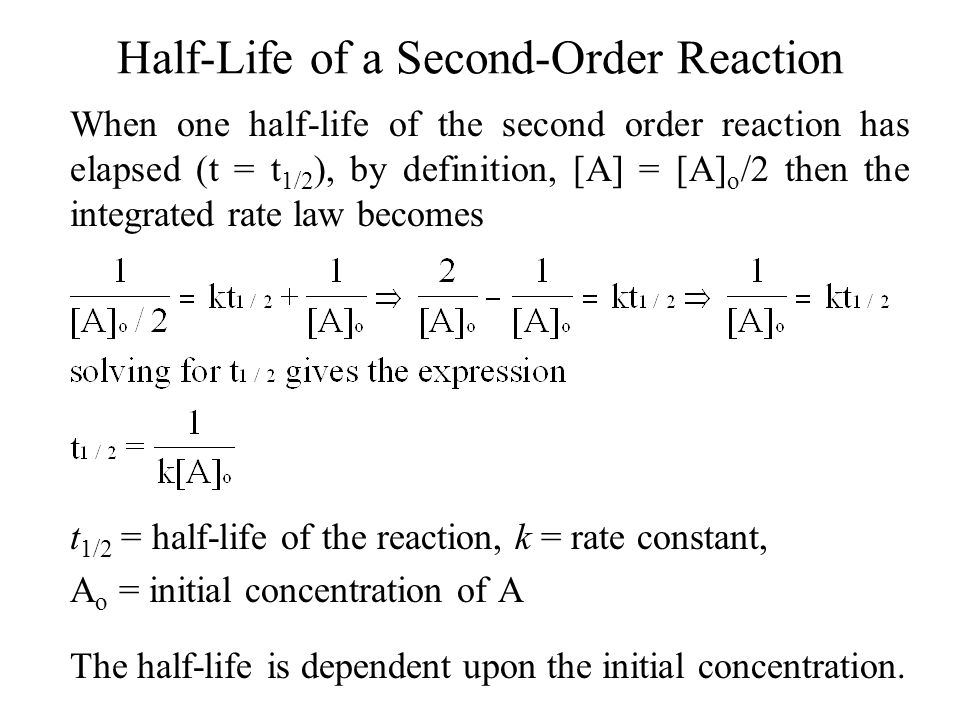
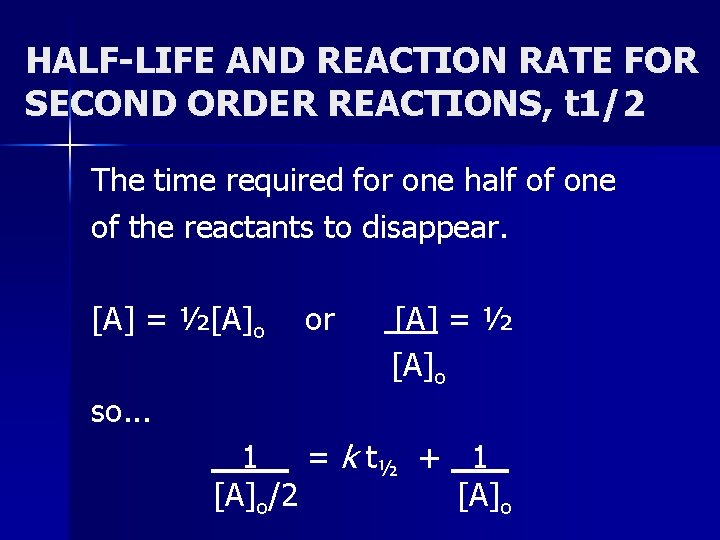
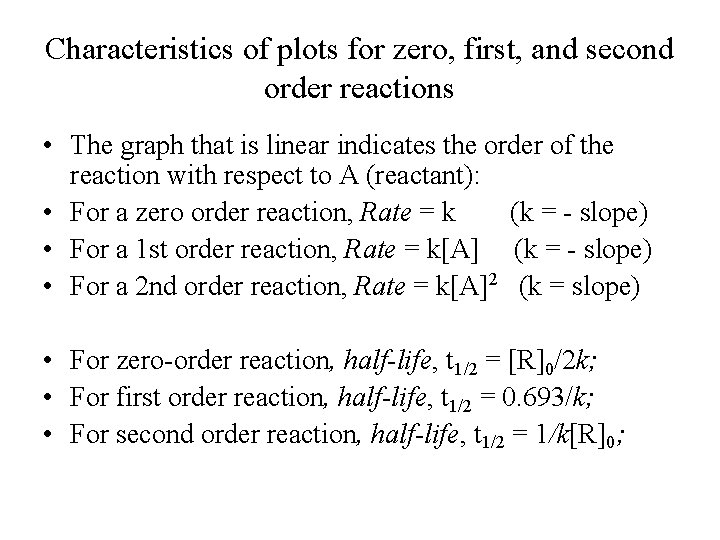
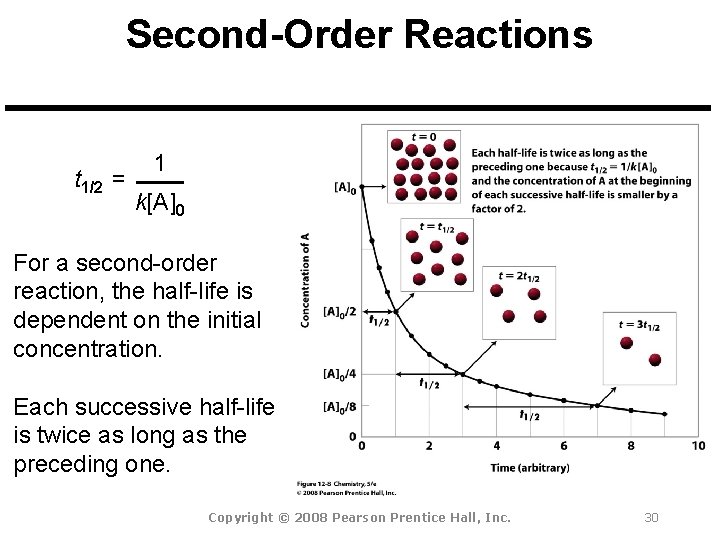
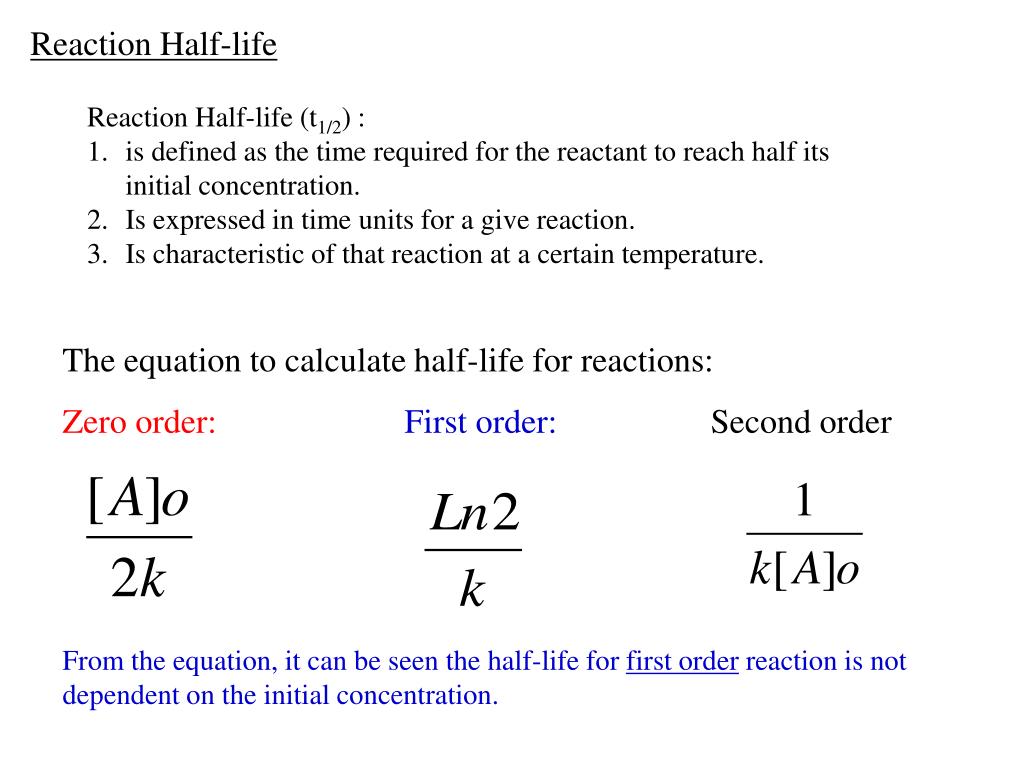
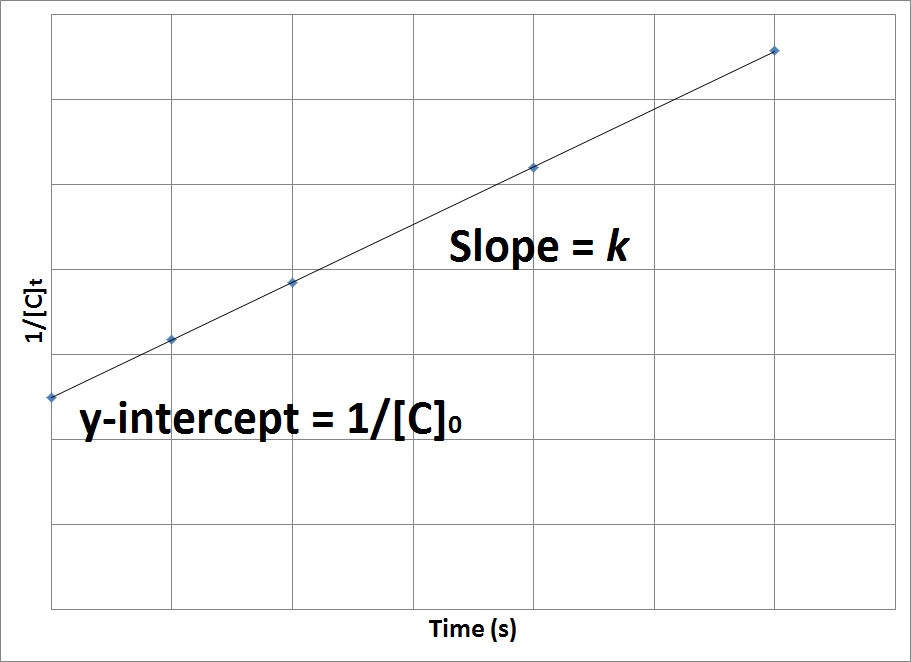
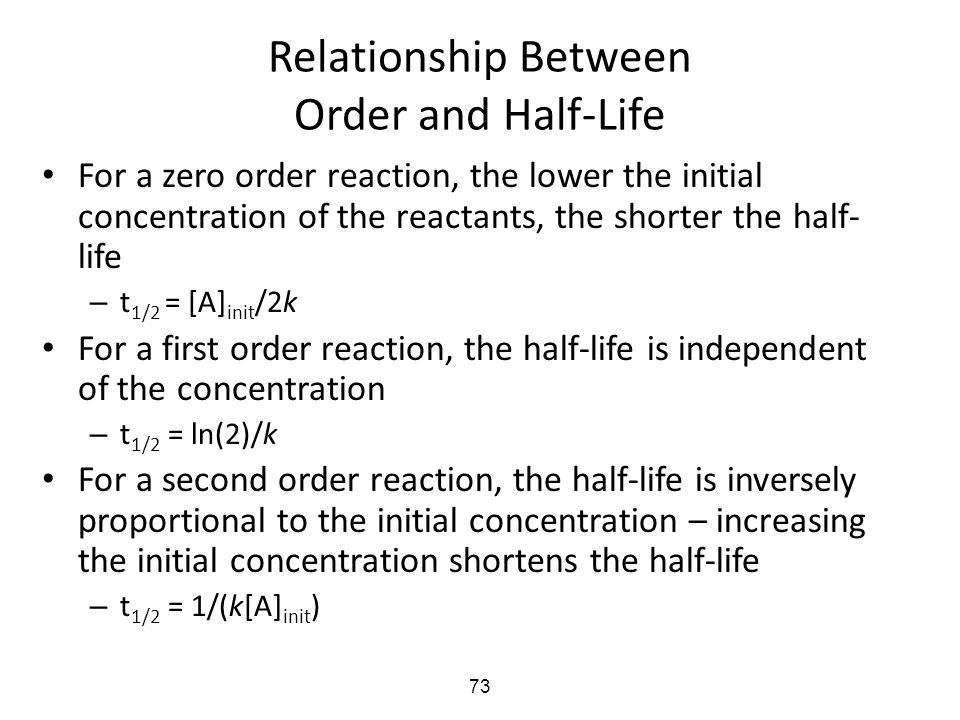
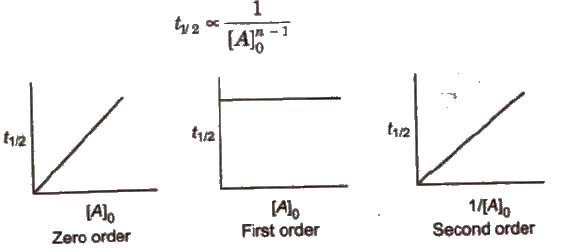
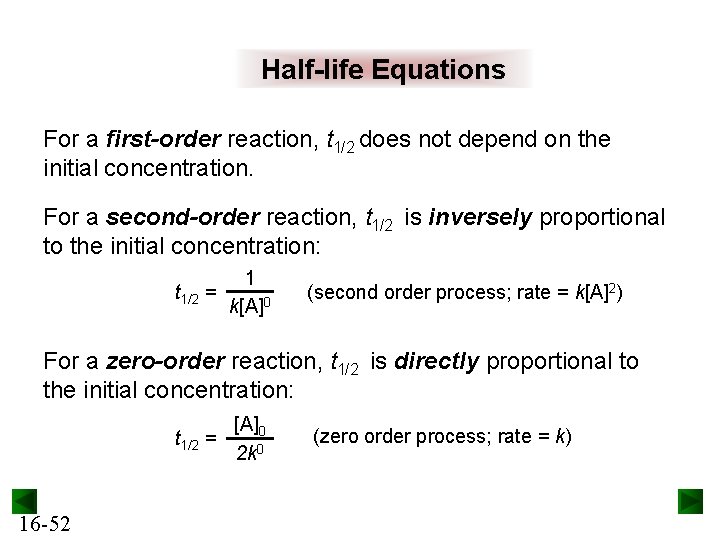
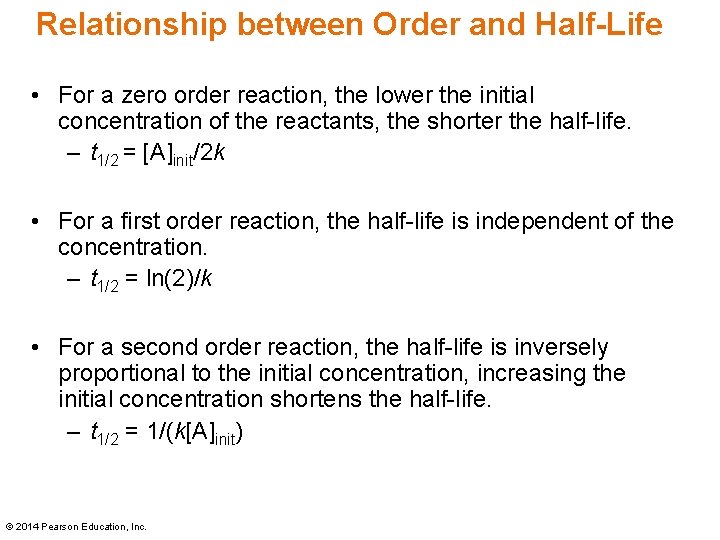
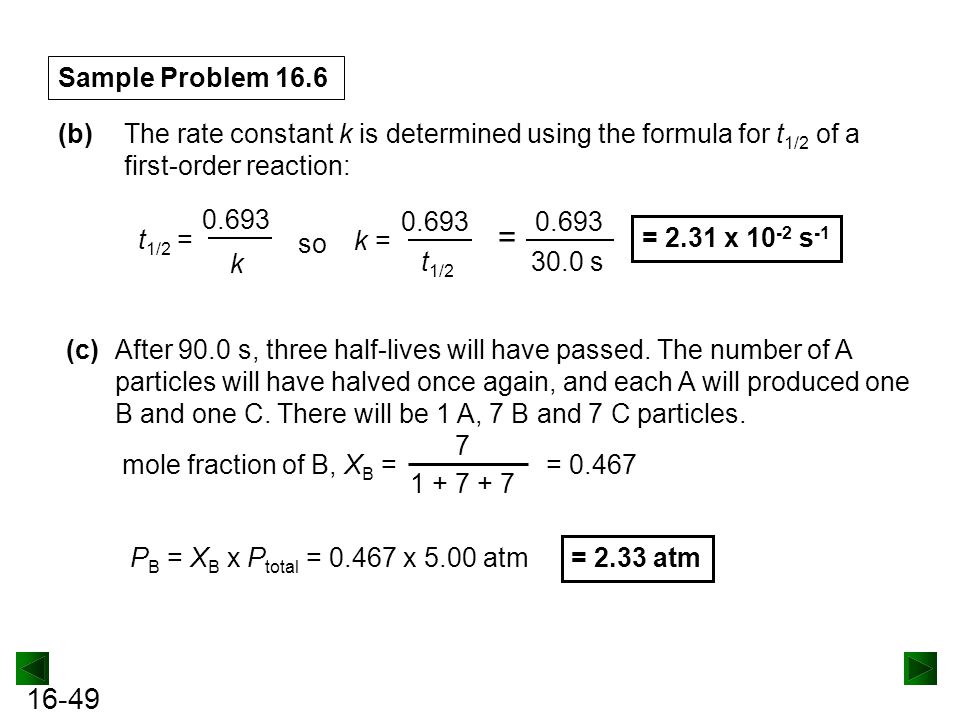
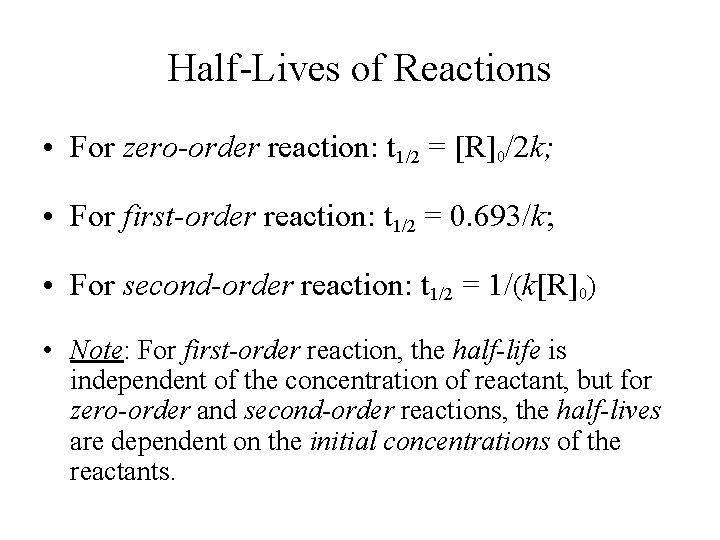
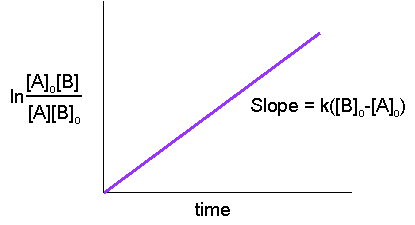
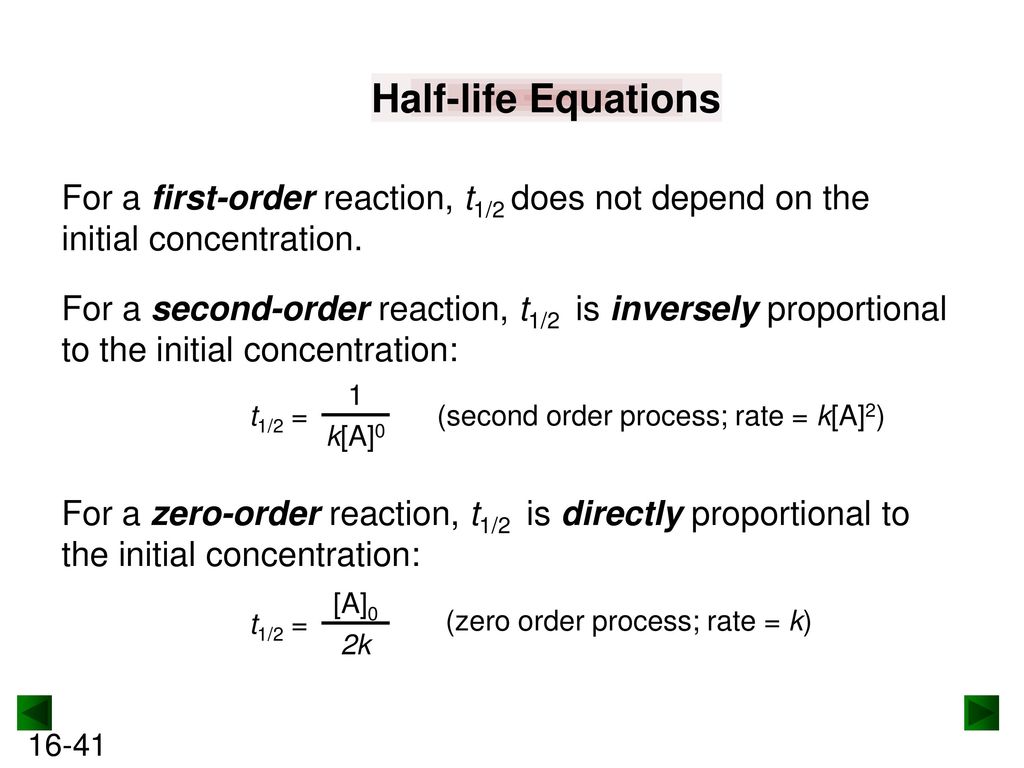
Secondorder reactions The integrated rate law for the secondorder reaction A → products is 1/ A_t = kt 1/ A_0 Because this equation has the form y = mx b, a plot of the inverse of A as a function of time yields a straight line The rate constant for the reaction can be determined from the slope of the line, which is equal to kFor a zero order reaction A products , rate = k t ½ = A o / 2k For a first order reaction A products , rate = kA t ½ = 0693 / k For a second order reaction 2A products or A B products (when A = B), rate = kA 2 t ½ = 1 / k A o Top Determining a Half LifeFor 1st order t1/2 ~ A ^0 For 2nd order t1/2 ~ A^1 Therefore, For nth order t1/2 ~ A^1n or t1/2 ~ 1/A^n1 Hope it helped My own answer Upvote if it helps
a zero order reaction b Pseudo zero order reaction c First order reaction d Second order reaction Answer a 3 Which of the following is the half life of zero order reaction? Two order reactions have half lives in the ration Then the ratio of time intervals , will be gt Where is the time period for completion completion of the first reaction and is time required for completion of the second reactionT1/2=0693/k For a secondorder reaction, the halflife depends on the rate constant and the concentration of the reactant and so is expressed as t1/2= 1/kA 1 A certain firstorder reaction (A> Productus) has a rate constant of 7×103 s^1 at 45 degrees celsius



Half Life Of A Second Order Reaction Derivation Youtube




Chapter 14 Chemical Kinetics 2 Chapter Goals 1 The Rate Of A Reaction Factors That Affect Reaction Rates 2 Nature Of The Reactants 3 Concentrations Ppt Download
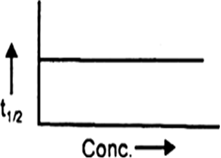
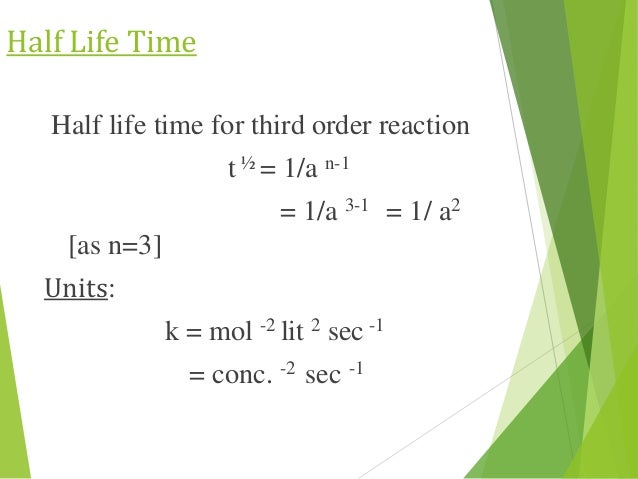
order reaction, the relationship between the half life period and the concentration is t 1/2 = ka n−1 1 But t 1/2 = ka 1 Hence, n−1=1 or n=2 Hence, for a second order reaction, the half life period is inversely proportional to the concentration Since the reaction order is second, the formula for t1/2 = k1A o1 This means that the half life of the reaction is seconds This means that Example 2 A reaction 2A > P has a second order rate law with k = 35E4 L/mols Calculate the time required for the concentration of A to change from 0260 mol/L to 0011 mol/L T1/2= 1/ka T1/2=0024min REFERENCE This topic has been referred from the following links 1




Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 0s 1 B 2 Mi N 1 C 4years 1




Solved Question 12 For A Second Order Reaction The Chegg Com
For a first order reaction, show that time required for 99% completion is twice the time required for the completion of 90% of reaction Q Calculate the osmotic pressure in pascals exerted by a solution prepared by dissolving 10 g of polymer of molar mass 185,000 in 450 mL of water at 37°CThe order of the reaction is second, and the value of k is M – 2 s – 1Since the reaction order is second, the formula for t1/2 = k1A o – 1This means that the half life of the reaction is seconds If t1/2 vs 1/a2 is a straight line graph then a determine the order of reaction (a) Zero order (b) First order (c) Second order (d) Third order




Second Order Reaction Definition And Derivation For Rate Law And Half Life



What Is The Ratio Of T3 4 T1 2 For A First Order Reaction Chemistry Topperlearning Com Qfujetmm
Medium View solution > The rate constant and half life of a first order reaction are related to each other as Medium View solution > A first order reaction takes 4 0 min for 3 0 % decomposition Calculate t 1 / 2In order to determine the rate law for a reaction from a set of data consisting of concentration (or the values of some function of concentration) versus time, make three graphs A versus t (linear for a zero order reaction) ln A versus t (linear for a 1 st order reaction) 1 / A versus t (linear for a 2 nd order reaction) Zero order reaction 2 First order reaction 3 Second order reaction 4 Third order reaction & Higher reaction is observed then graph of 1/ax vs t gives straight line with slope K and intercept 1/k



Chapter 12 Chemical Kinetics Ppt Video Online Download



The Following Graph Is A Plot Of T1 2 And Concentration What Is The Order Of The Reaction From Chemistry Chemical Kinetics Class 12 Cbse
A 693 s1 b 0693 s1 c s1 d 0144 s1 e 301 s1 Chemistry The rate constant for a secondorder reaction is 054 M1s1 What is the halflife of this reaction if the initial concentration is 027 MWhat statement below best describes the graph representing the integrated secondorder rate law?A t1/2 = A0 /2k b t1/2 = 0693/2k c t1/2 = A0 /2 d t1/2 = 2k/ A0 Answer a 4 The unit of k for zero order reaction is a moles/litre/second b moles c moles
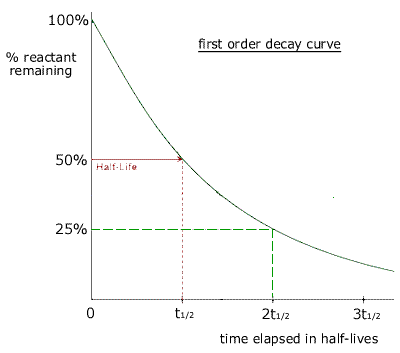



Kinetics 6 34 Half Life
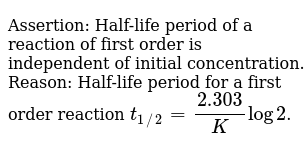



Assertion Half Life Period Of A Reaction Of First Order Is Independent Of Initial Concentration Reason Half Life Period For A First Order Reaction T 1 2 2 303 K Log 2
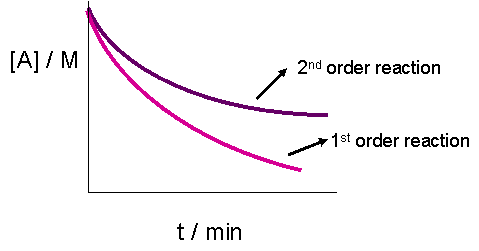
A plot of A versus t for a zeroorder reaction is a straight line with a slope of −k and a yintercept of A 0shows a plot of NH 3 versus t for the thermal decomposition of ammonia at the surface of two different heated solids The decomposition reaction exhibits firstorder behavior at a quartz (SiO 2) surface, as suggested by the exponentially decaying plot of concentration versus timeAug 24,21 For a second order reaction, 2 A → Products a plot of log t1/2 vs log a (Where a is the initial concentration) will give an intercept equal to which of the following ?a)1/Kb)c)d)log KCorrect answer is option 'D' Can you explain this answer?For second order reaction, t 1/2 ∝ 1/A 0 Integrated rate law expression For Zeroth order reaction A → product Initially a o at t = t 1 (a x) x The rate of reaction at time 't'




The Concept Of T1 2 Is Useful For The Reactions Of



Chemical Kinetics The Rates And Mechanisms Of Chemical
What is the general formula for a 2nd order chemical reaction?__3125% ____ What is the halflife for a first order reaction if the initial concentration of reactant is 1Get answer For a second order reaction, 2Ararr Products, a plot of log t_(1,,2) vs log a (where a is initial concentration) will give an intercept equal to which one of the following?




Iit Jee Rate Constant Half Life For Nth Order Reaction Lecture 15 Offered By Unacademy



Order Of A Reaction 2302
__1/At___ For a zero order reaction?Halflife equation for firstorder reactions t 1/2=0693/k where t1/2 is the halflife in seconds , and k is the rate constant in inverse secondsWhat is the halflife of a firstorder reaction with a rate constant of 0 x 10 4 s1?Examples of Zero Order Reaction The following reactions are examples of zero order reactions that are not dependent on the concentration of the reactants The reaction of hydrogen with chlorine (Photochemical reaction) H2(g)Cl2(g) hv → 2HCl(g) H 2 ( g) C l 2 ( g) → h v 2 H C l ( g) Decomposition of nitrous oxide over a hot platinum
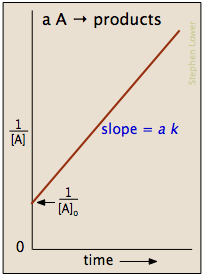



2 8 Second Order Reactions Chemistry Libretexts




Half Life Equation For First Order Reactions T1 2 0 693 K Clutch Prep
The plot of a concentration of the reactant versus time for a reaction is a straight line with a negative slope The reaction follows a first order rate equation zero order rate equation second order reaction third order rate equationFor n t h order reaction, the relationship between the half life period and the concentration is t 1 / 2 = k a n − 1 1Therefore you have to first find the rate constant by the equation T1/2= 0693/kand then put the value of rate constant (k) in integrated rate law equation ie, Ln (a°)/ (a)= k*T1/2 here a° = initial concentration ,a = final remainin Continue Reading It can be answered in two ways



Chapter 12 Chemical Kinetics Chemical Kinetics Studies The



Half Life For First And Second Order Reactions The Chegg Com
039% (divide by 2 eight times) What percentage of a sample remains after 5 half lives?Solution (the general way) 1) Find the rate constant ln A = kt ln A o ln 035 = (k) (65 hr) ln 0701 In a first order reaction 80% of the reactant at an instant was reduced to 8% in 4606 seconds The rate constant of the reaction is KEAM 15 2 t 1 / 2 for a first order reaction is 1426 m i n Calculate the time when 5 % of the reactant is left UP CPMT 15



Second Order Reaction



Chemical Kinetics Expression Of Rates Stoichiometric Relationships Of
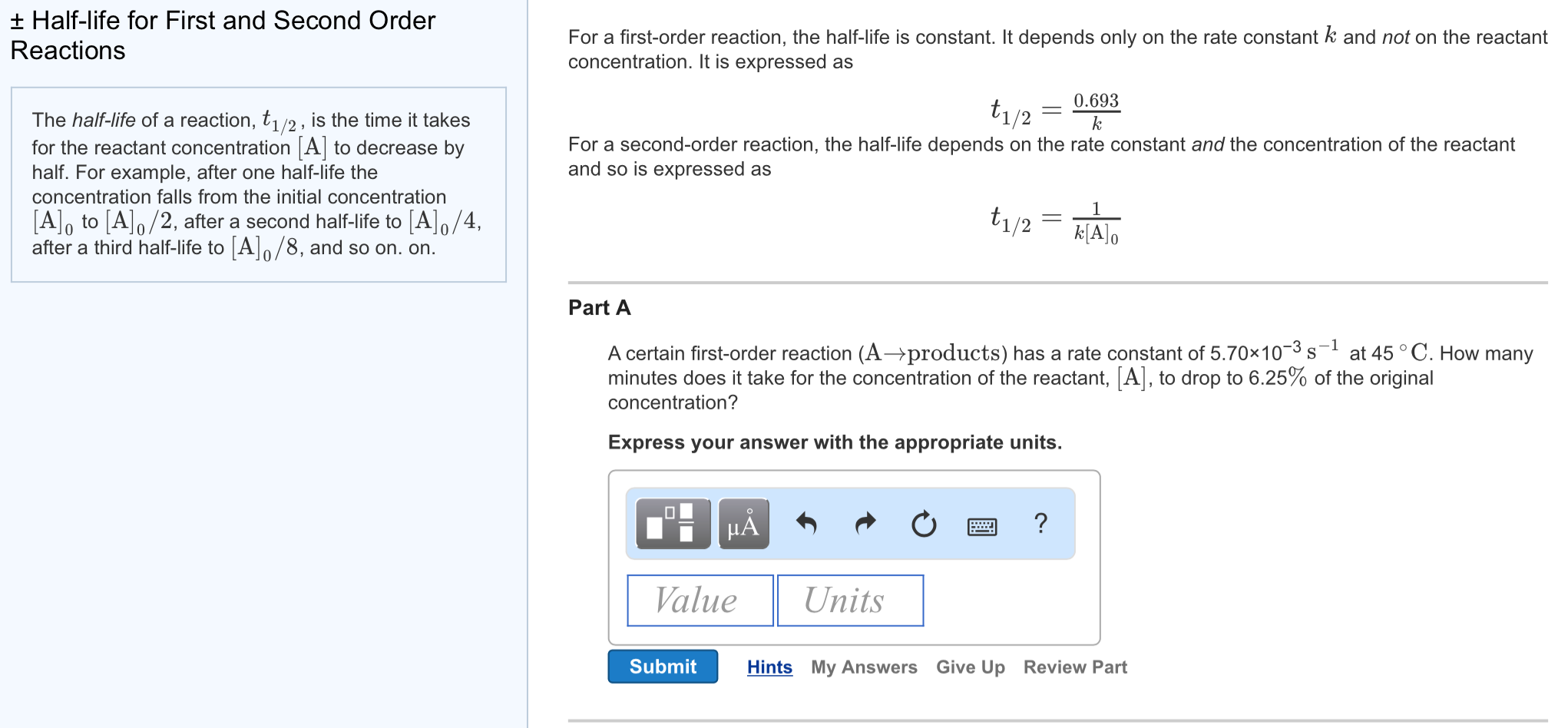
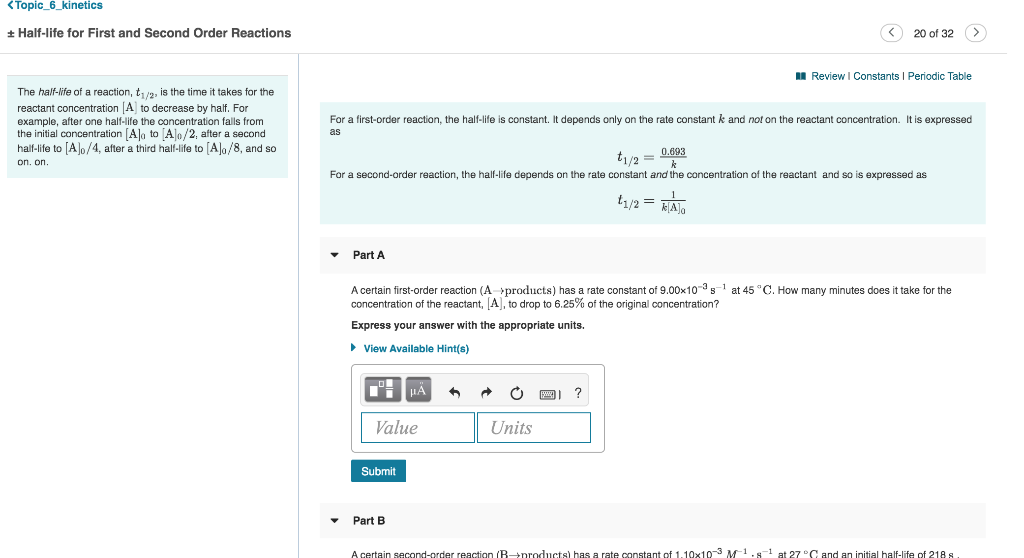
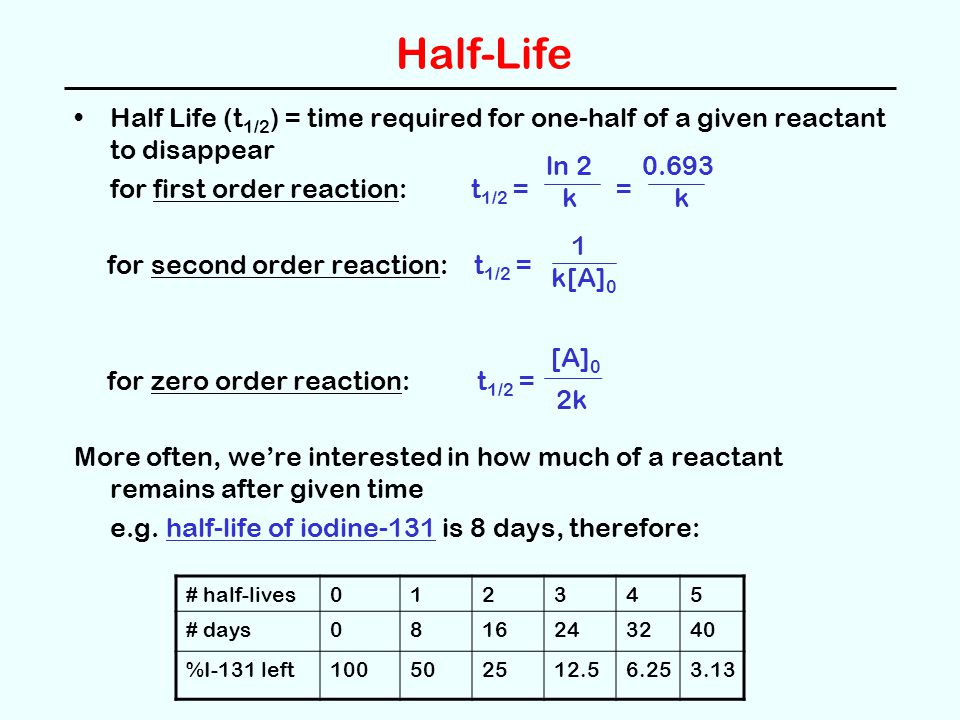
Solution for For a secondorder reaction, the halflife is equal to a) t1/2=0693/k b) t1/2=k/0693 c) t1/2=1/kAo d) t1/2=k e) t1/2=A}o/2kSolution for (a) A reaction is second order in A and first order in B(i) Write the differential rate equation,(ii) How is the rate affected on increasing theThe halflife of a reaction (\(t_{1/2}\)), is the amount of time needed for a reactant concentration to decrease by half compared to its initial concentration Its application is used in chemistry and medicine to predict the concentration of a substance over time
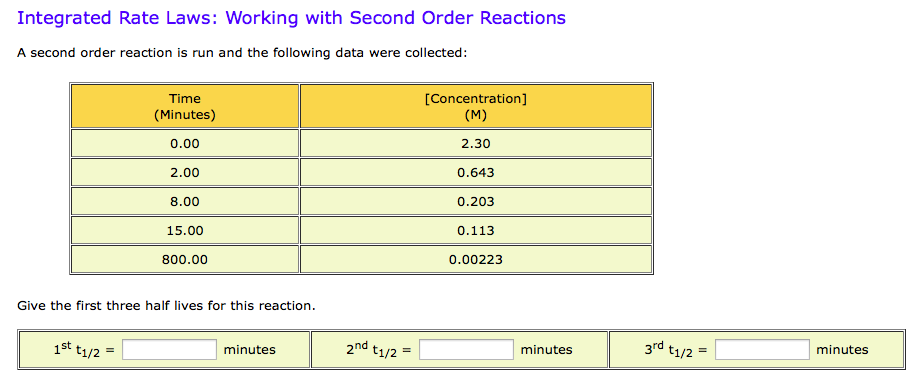



Integrated Rate Laws Working With Second Order Chegg Com




The Half Life Of A Reaction T1 2 Is The Time It Takes For The Reactant Concentration A Homeworklib
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How works Test new features Press Copyright Contact us Creators A first order reaction takes 10 min for 25% decomposition calculate t1/2 for the reaction 2 See answers Advertisement Advertisement topanswers topanswers Given Time = 10 min Decomposition = 25 % To find Half life period Solution For a first order reaction, k = 2303 / time log ( R ( 0 ) / R ) Where, R ( 0 ) Initial concentration R The rate constant for a secondorder reaction is 054 M1s1 What is the halflife of this reaction if the initial concentration is 027 M Chemistryrate laws 1 Determine the overall reaction order for the reaction whose rate law is as follows rate=kO3ClO a 0




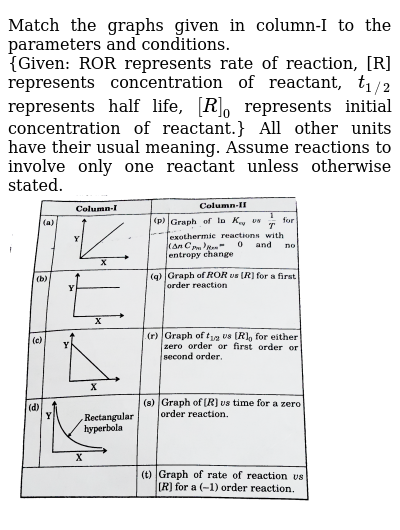
Column I Column Ii P Zero Order Reaction 1 T



John E Mc Murry Robert C Fay C
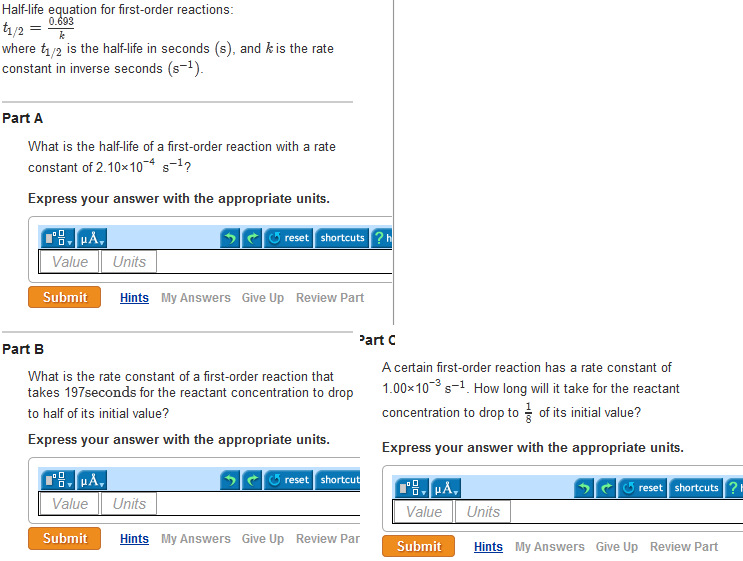
For a second order reaction, `2Ararr` Products, a plot of log `t_(1//2)` vs log a (where a is initial concentration) will give an intercept equal to which onWhat is second order reaction with example, what is second order reaction give example, what is second order reaction, what is 2nd order reaction, define second order reaction with exampleHalf life equation for first order reactions t1/2 = 0693/kwhere t1/2 is the half life in second and k is the rate constant ininverse seconds A what is the half life of a first order reaction with a rateconstant of 510 * 10^4/s




Half Life Of A First Order Reaction Video Khan Academy



Ppt Chapter 16 Powerpoint Presentation Free Download Id
EduRev NEET Question is disucussed on EduRev Study Group by 185 NEET StudentsT1/2 for first order reaction is (a) 06/k (b) 0693/k (c) 06/k (d) 010/kProblem #7 The decomposition of aqueous hydrogen peroxide to gaseous oxygen and water is a firstorder reaction If it takes 65 hours for the concentration of H 2 O 2 to decrease from 070 to 035, how many hours are required for the concentration to decrease from 040 to 010 ?



Solved Half Life For First And Second Order Reactions Chegg Com



Integrated Rate Law
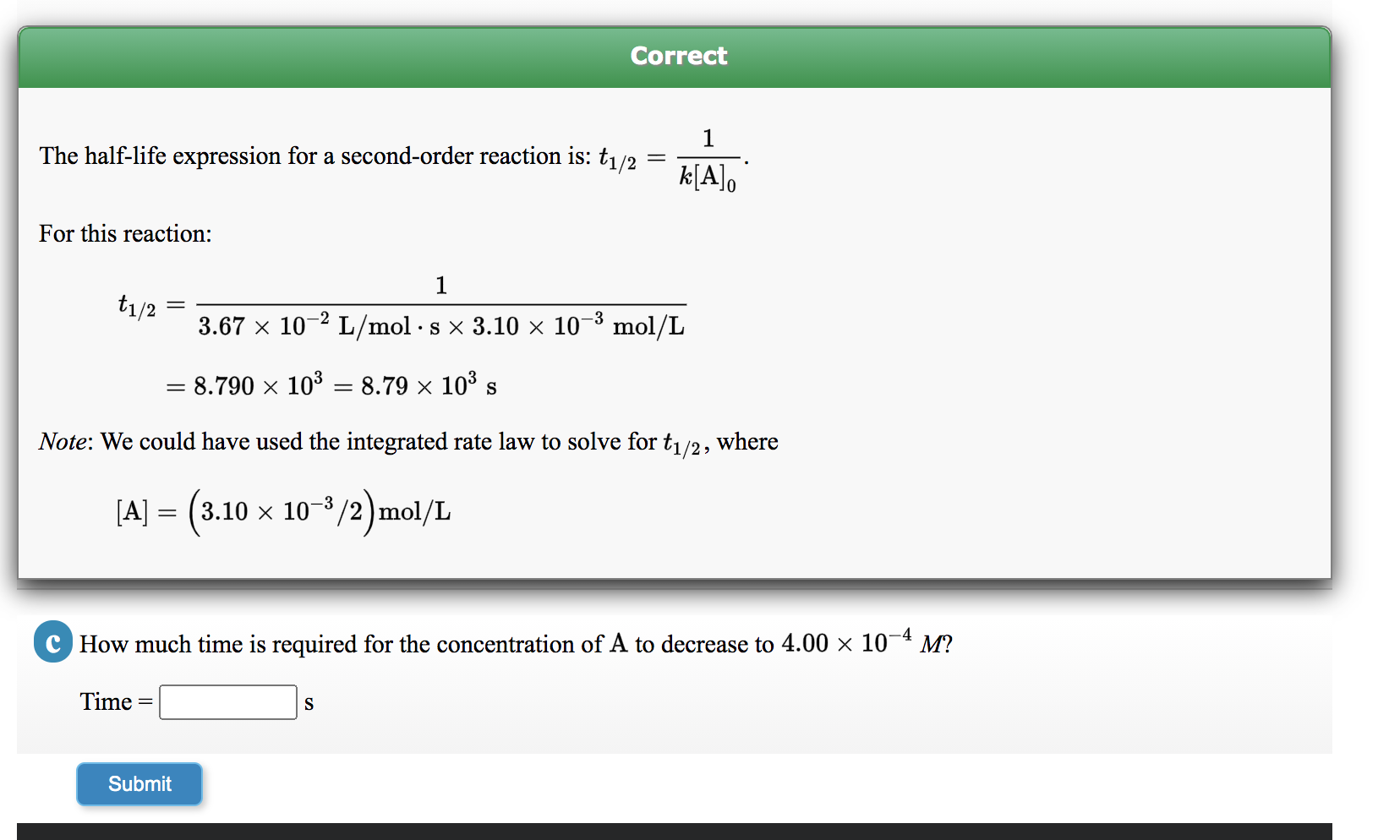
Question Halflife for First and Second Order Reactions 11 of 11 The halflife of a reaction, t1/2, is the time it takes for the reactant concentration A to decrease by half For example, after one halfMe the concentration falls from the initial concentration (Alo to A\o/2, after a second halflife to Alo/4 after a third halflife to A/8Remember halflife is symbolized by t 1/2 The halflife is the time that it takes for the concentration of a reactant to decrease to half of its initial concentration We've also already talked about the integrated rate law or the integrated rate equation for a second order reaction So here's one form of5 rows The value of t1/2 for second order reaction is A 1/ak B 0693/k C Ao/2k D Ao/2 Answer»



Solved The Half Life Of A Reaction T1 2 Is The Time It Chegg Com




Determination Of Order Of Reaction Pdf



Chm 1046



Concentration Time Relationships Integrated Rate Laws Introductory Chemistry 1st Canadian Edition



Chapter 13 Chemical Kinetics Ppt Download




Zero Order Reaction Definition Derivation Graph Examples
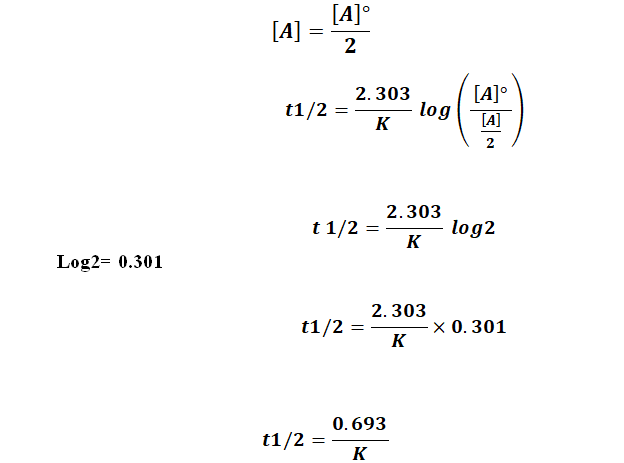



Half Life Formula




If The Half Life Period Of A First Order Reaction In A Is 2 Minutes How Long Will It Take A To Reach 25 Of Its Initial Concentration




Answered The Half Life Of A First Order Reaction Bartleby



Half Life Ppt Download




Solved 10 The Rate Constant For A Second Order Reaction Is Chegg Com



1




Chemical Kinetics By Prof Sanaa Taher Arab Physical Chemistry Ppt Download



Cbse Class 12 Chemistry Notes Chemical Kinetics Aglasem Schools




Half Life T 1 Of The First Order Reaction And Half Life T 2 Of The Second Order Reaction Are Equal Hence Ratio Of The Rate At The Start Of The Start Of The Reaction




Solved Numerical Based Ratio Of Tza And T1 2 For A Second Order Reaction Involving Single Reactant Find The Ratio Of T3 4 And Following Data Of Initial Concentrations And Raton Nolaule



Chapter 16 Kinetics Rates And Mechanisms Of Chemical



Solved Correct 1 The Half Life Expression For A Second Order Chegg Com




Find The Ratio T7 8 T1 2 For First Order Reaction Brainly In




Solved A Reaction Is First Order In A If The Rate Constant Chegg Com




Solved For A First Order Reaction The Half Life Is Chegg Com



Chapter 13 Lecture Presentation Chapter 13 Chemical Kinetics




For A Second Order Reaction 2ararr Products A Plot Of Log T 1 2 Vs Log A Where A Is Initial Concentration Will Give An Intercept Equal To Which One Of The Following Img




Chem 2 Chemical Kinetics V The Second Order Integrated Rate Law



Reaction Rates Chapter 13 Ppt Video Online Download



What Is The Order If Half Life Of A Reaction Is Halved As The Initial Concentration Of The Reactant Is Doubled Quora




Zero Order Reactions Study Material For Iit Jee Askiitians



2 8 Second Order Reactions Chemistry Libretexts




First Order Reaction An Overview Sciencedirect Topics



What Is The Relation Between T1 2 And T3 4 For Zero Order Reaction Quora



Chapter 16 Kinetics Rates And Mechanisms Of Chemical Reactions Ppt Download




The General Expression For Half Life Period Of An Nth Order Reaction For N 1 Is



Chemical Kinetics Expression Of Rates Stoichiometric Relationships Of




Half Life Equation For First Order Reactions T1 2 0 693k Where T1 2 Is The Half Life In Seconds S And K Homeworklib




Solved Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 0 S 1 B 2 Min 1 C 4 Year 1




A First Order Reaction Has A Rate Constant Of 1



2




Half Life T1 Of The First Order Reaction And Half Life T2 Of The 2 Nd Order Reaction Are Equal Hence Ratio Of The Rate At The Start Of The Reaction




Solved What Is The Ratio Of T1 2 To T1 3 For A First Order Reaction



2




1 Chapter 12 Chemical Kinetics 1 Second Order Rate Law 2 Zero Order Rate Law 3 Reaction Mechanism 4 Model For Chemical Kinetics 5 Collision 6 Catalysis Ppt Download



What Is Half Life Of A First Order Reaction Quora




For A Zero Order Reaction The Relationship Between T1 2 1st And T1 2 2nd Is




Chemical Kinetics By Prof Sanaa Taher Arab Physical Chemistry Ppt Download




First Order Reactions Chemistry Class 12 Iit Jee Main Advanced Neet Aipmt Askiitians Youtube




What Is The Ratio Of T3 4 T1 2 For A First Order Reaction Chemistry Topperlearning Com Qfujetmm



Solved Half Life Equation For First Order Reactions Chegg Com




Half Life Introduction To Chemistry



The Following Graph Shows How T 1 2 Half Life Of A Reactant R Changes With The Initial Reactant Concentration A 0 The Order Of Reaction Will Be Img Src D10lpgp6xz60nq Cloudfront Net Physics Images Din Obj Chm V01 C3 3 E01 254 Q01 Png




Half Life Equation For First Order Reactions T1 2 0 693 K Clutch Prep



2 8 Second Order Reactions Chemistry Libretexts
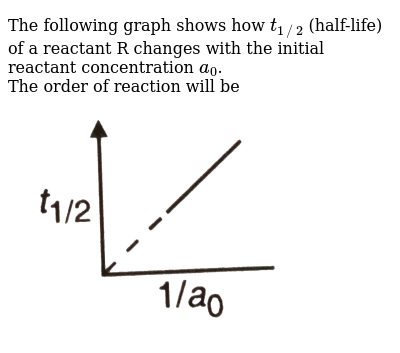



The Following Graph Shows How T 1 2 Half Life Of A Reactant R Changes With The Initial Reactant Concentration A 0 The Order Of Reaction Will Be Img Src D10lpgp6xz60nq Cloudfront Net Physics Images Din Obj Chm V01 C3 3 E01 254 Q01 Png



14 6 Second Order Reactions Chemistry Libretexts



Half Lives




For A Second Order Reaction 2ararr Products A Plot Of Log T 1 2 Vs Log A Where A Is Initial Concentration Will Give An Intercept Equal To Which One Of The Following Img




A Zero B First C Second Order Reaction Kinetics For Degradation Download Scientific Diagram




Half Lives Chemistry Classroom Teaching Chemistry Chemistry Experiments



Chapter 16 Kinetics Rates And Mechanisms Of Chemical Reactions Ppt Download




2 Chemistry Part Ii Pages 151 0 Flip Pdf Download Fliphtml5



Half Life Of A Second Order Reaction Video Khan Academy



1




Second Order Reaction An Overview Sciencedirect Topics




The Half Life For The Second Order Decompo Clutch Prep
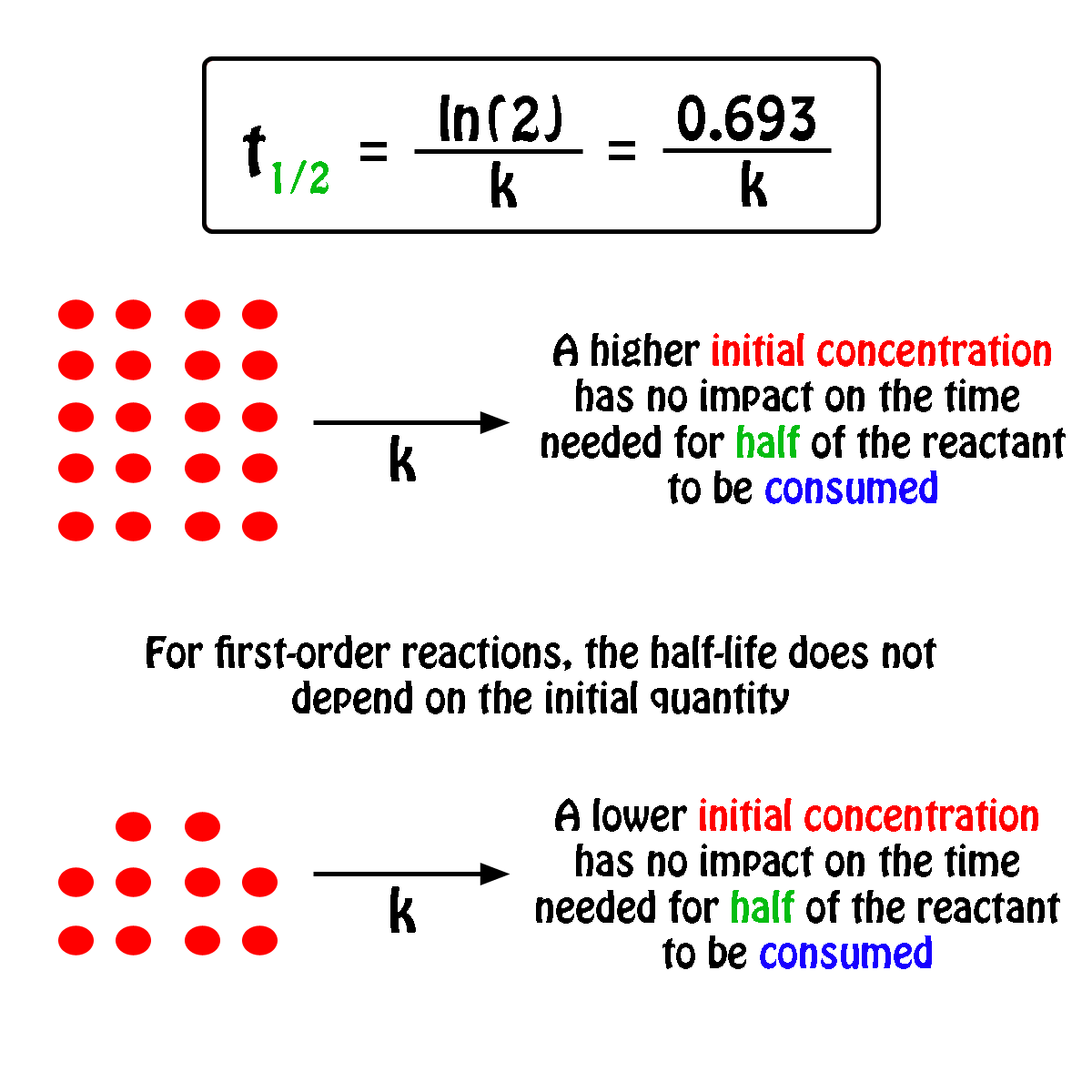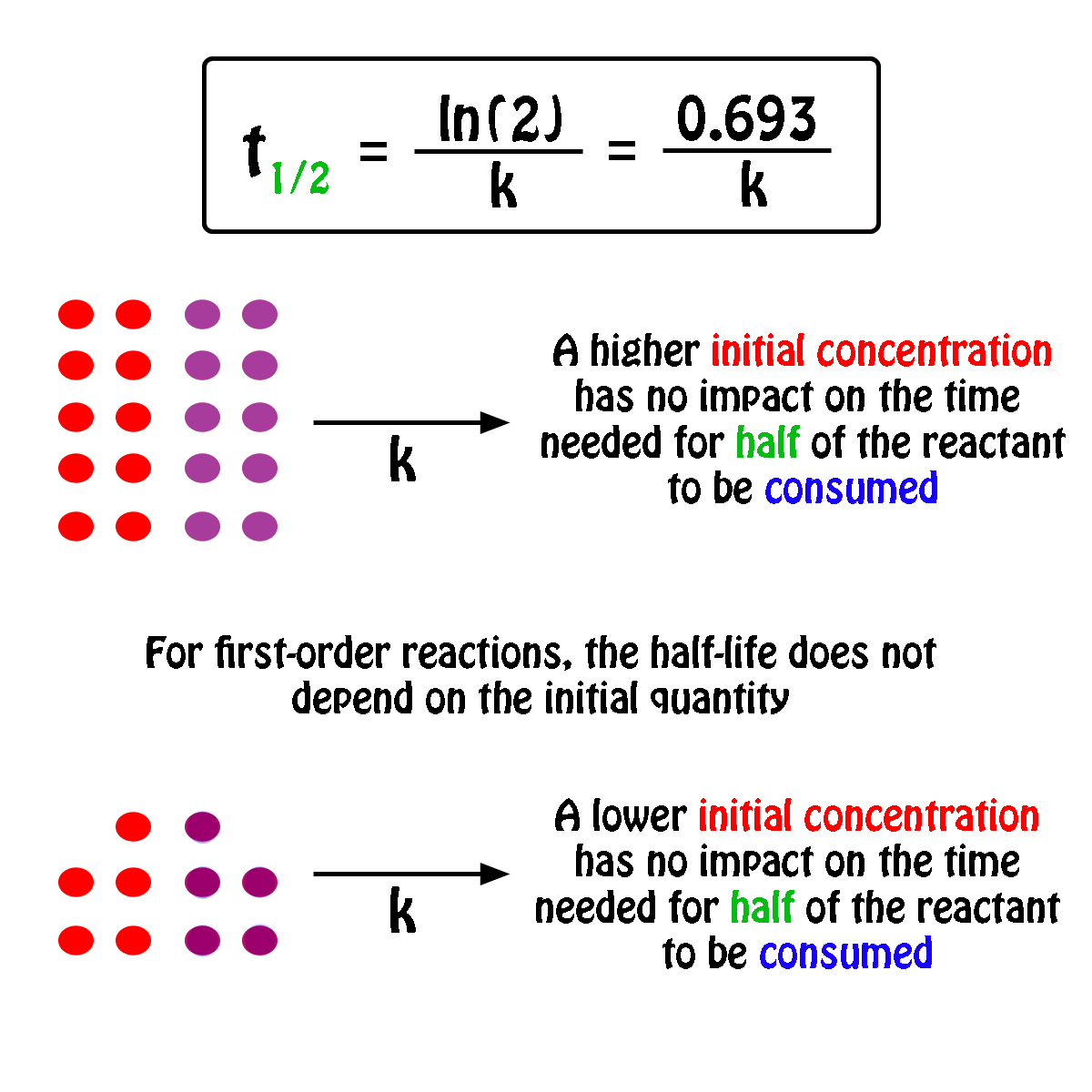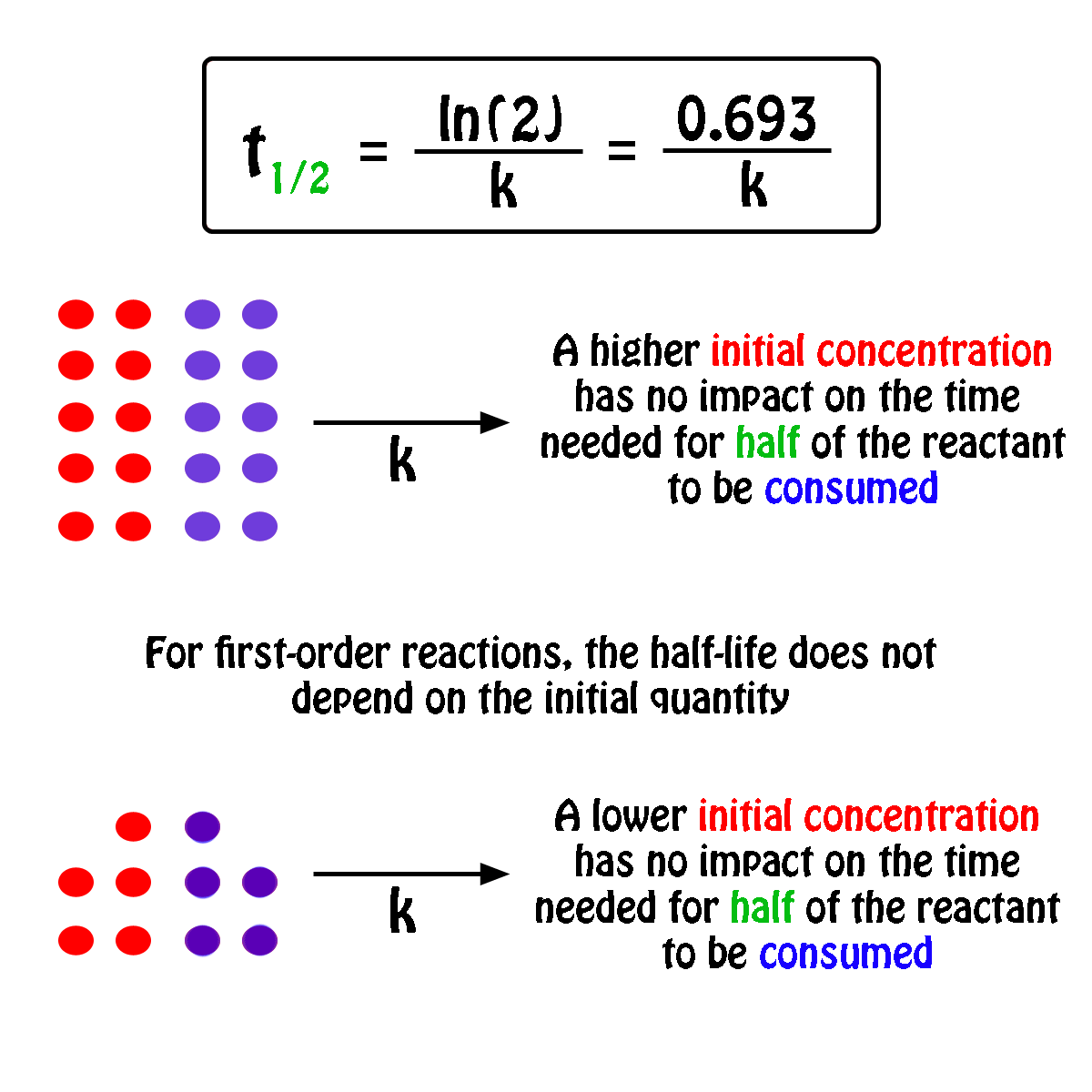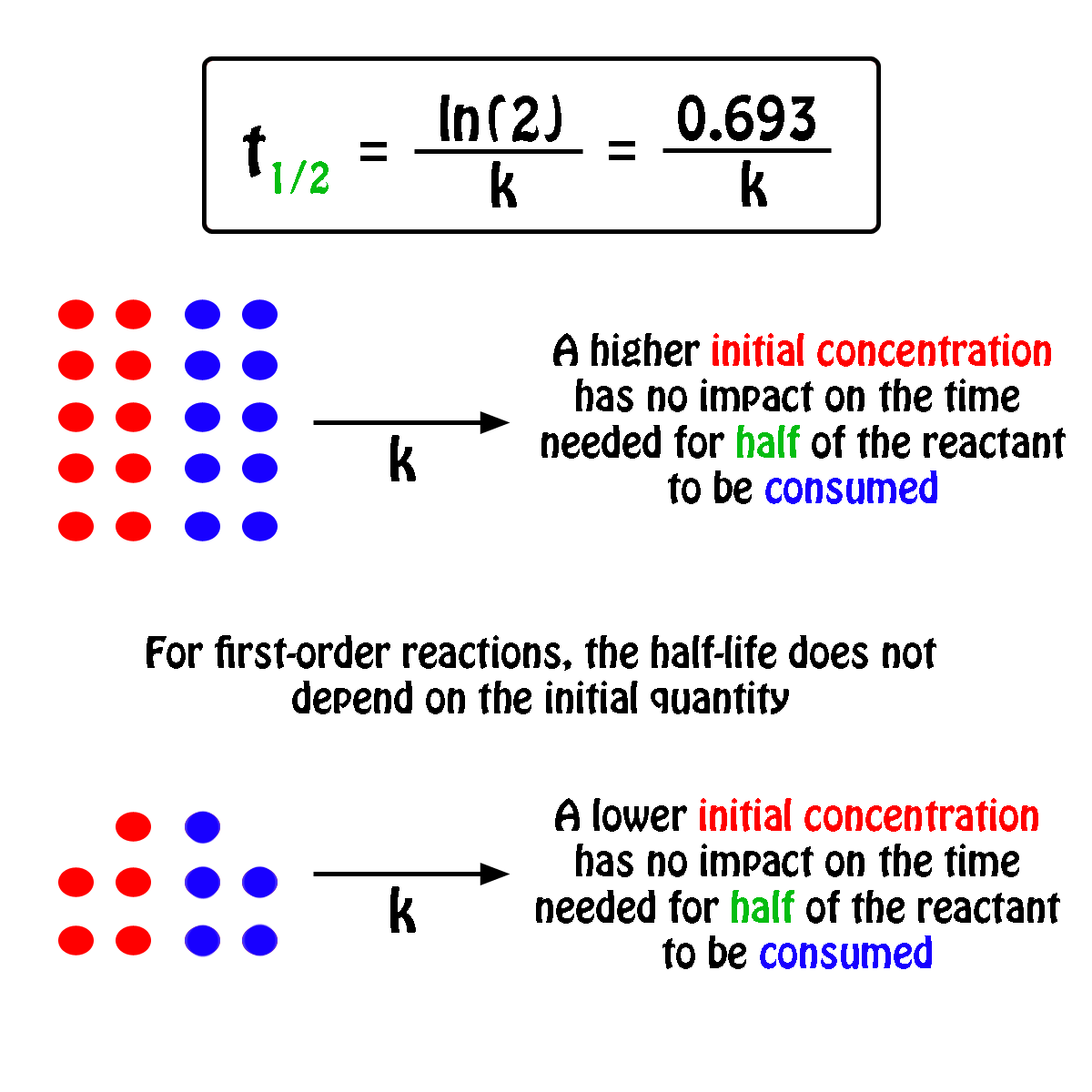



What Is The Half Life Of A First Order Reaction With A Rate Constant Of 7 80xx10 4 S 1 Socratic




Solved For A Second Order Reaction 2a Products A Plot Of Logt1 2 Vs Loga Where A Is Initial Concentration Will Give An Intercept Equal To Which One Of The Following




Ppt Summary Of The Kinetics Of Zero Order First Order And Second Order Reactions Powerpoint Presentation Id



If Half Period Of A Reaction T1 2 Is Doubled As The Initial Concentration Of Reactant Is Also Doubled How Do You Evaluate The Order Of Reaction Quora




Whar Happens To The Half Life T1 2 Of A Secondorder Reaction As The Substabce Decays Concentration Homeworklib
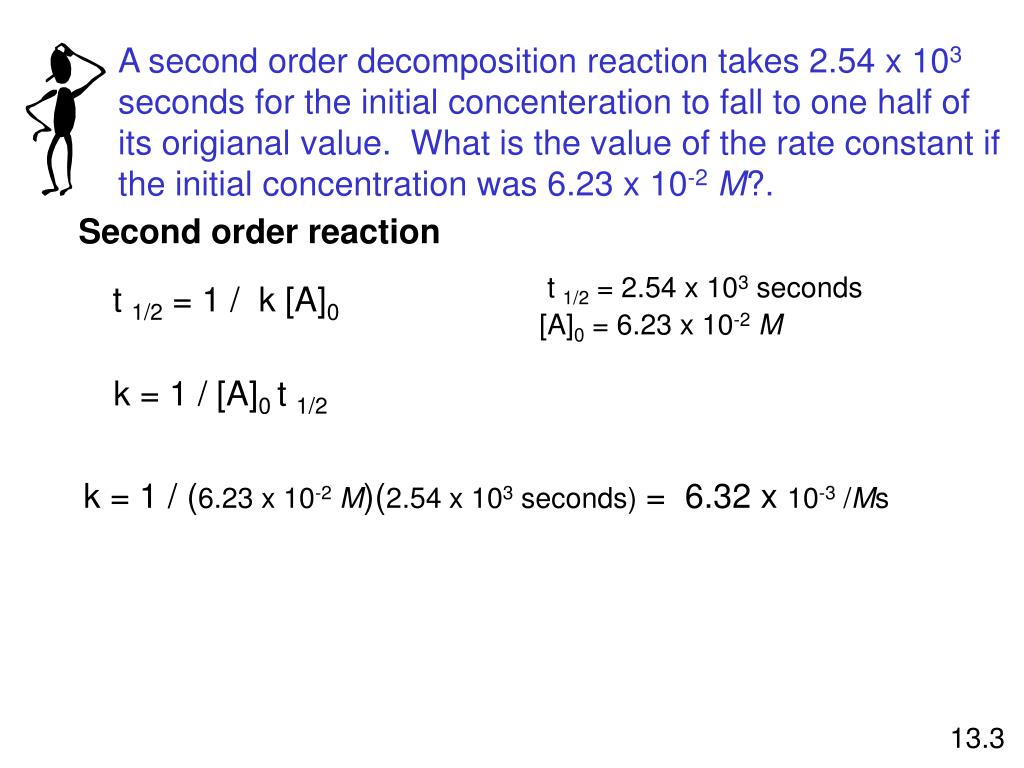



Ppt Summary Of The Kinetics Of Zero Order First Order And Second Order Reactions Powerpoint Presentation Id



For A Certain First Order Reaction T1 2 100 Sec How Long Will It Take For The Reaction To Complete 75 Quora
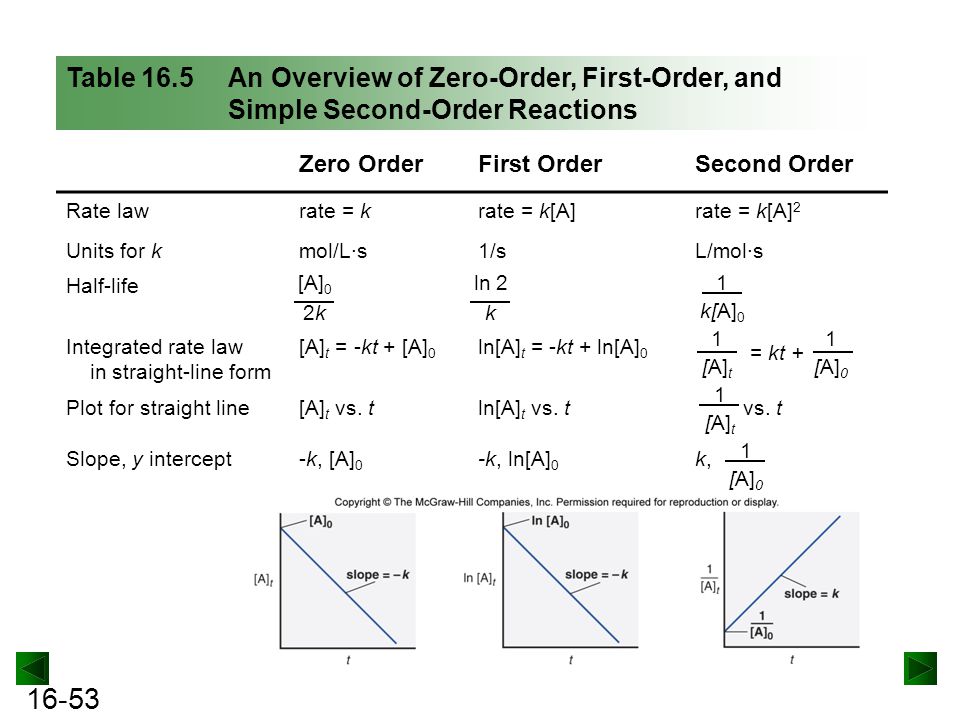



Chapter 16 Kinetics Rates And Mechanisms Of Chemical Reactions Ppt Download




Using The First Order Integrated Rate Law And Half Life Equations Worked Example Video Khan Academy
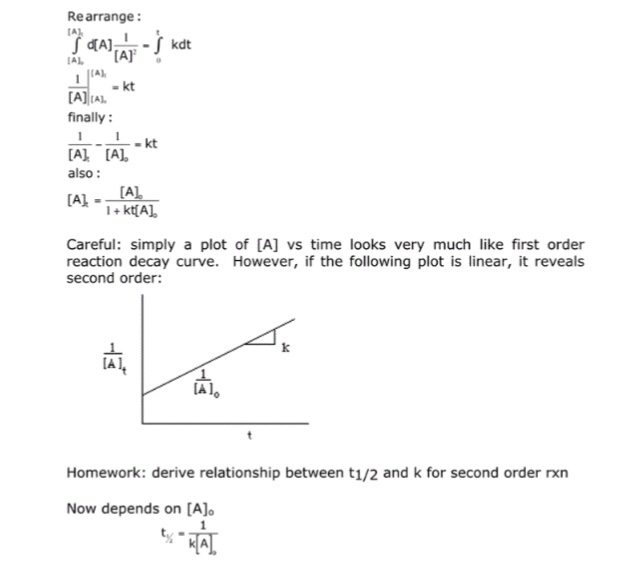



Solved Derive An Relationship Between T1 2 And K For A Chegg Com
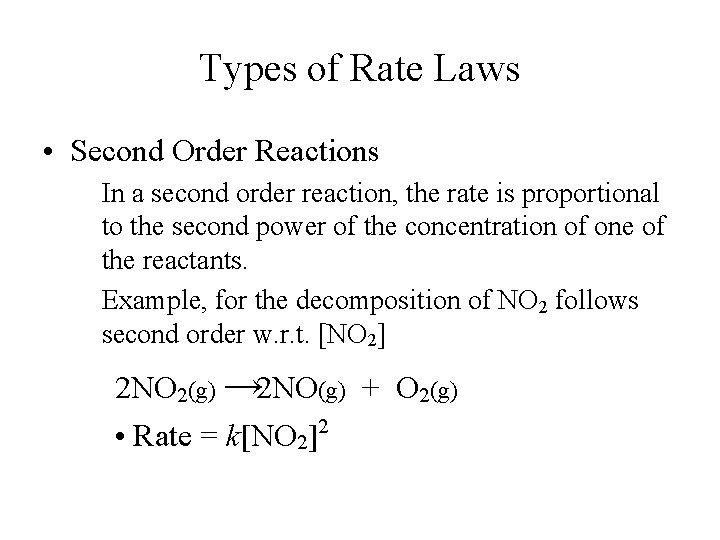



Chemical Kinetics Expression Of Rates Stoichiometric Relationships Of



What Is The Meaning Of Concentration Of Reactant




Rate Equation Wikipedia



0 件のコメント:
コメントを投稿